

How modern sensors improve bioprocess development in shake-flask experiments
With the ability to conduct multiple experiments at the same time, shake flasks are a researcher’s best friend when it comes to efficient and versatile microbial screening. Here, though, sbi’s Tiffany Fredette explains how they have come into the digital age
Shake flasks have long been considered a cornerstone vessel type in bioprocessing. Although invaluable tools in bioprocessing, they do have limitations compared to bioreactors, particularly in industrial-scale production and certain research applications. Traditionally, shake -flask cultures have relied on manual handling, periodic monitoring, and manual adjustment of environmental parameters by technicians. But this process of manual sampling is time-consuming and invasive, resulting in low-resolution data with additional risks such as contamination.

In recent years, the integration of automation technologies into shake-flask bioprocessing represents a significant advancement in the field of biotechnology, bridging the gap between traditional manual techniques and the precision of modern bioreactor systems. New, modern sensor technologies allow scientists to study key parameters in shake flasks non-invasively, automatically, and in real-time. Automation has revolutionized shake-flask bioprocessing, offering enhanced reproducibility, scalability, and efficiency in both research and industrial settings.
Process optimization in Pichia Pastoris: a case study
Dr Christina Dickmeis, a Senior Research Scientist at Scientific Bioprocessing Inc (sbi), sought to optimize protein production in the expression host, Pichia Pastoris. A critical aspect of P. pastoris expression systems, methanol induction enables efficient and controlled expression of recombinant proteins. However, proper methanol induction can pose challenges that are difficult to overcome via manual methods.
Early tests used the Cell Growth Quantifier (CGQ) and Liquid Injection System (LIS) by sbi in which improvements using biomass-based feeding were made to the process. The CGQ, an optical sensor placed beneath the shake flask, monitored biomass non-invasively through the vessel wall. Once cell growth on the primary substrate (glycerol) was completed and sufficient biomass was generated, indicated by a reduction in cell growth rate, the LIS was used to initiate methanol induction. The use of these sensors allowed for process optimization including the testing of different feeding strategies, amount of methanol feed, and adaptation timeframes to find out which induction approach would result in the highest protein yield. Ultimately, the step-by-step optimization performed with the CGQ and LIS system achieved a tenfold increase in product yield. This ‘blind’ shake-flask optimization was performed over 200 single shake-flask experiments and ultimately required three quarters of a year to accomplish.
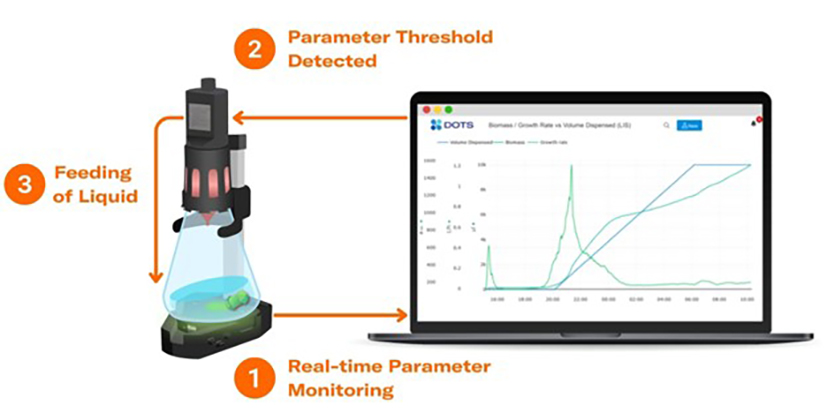
Culture monitoring with parameter-based feeding: a new application
The automated feeding of substrates – an operation that has been routinely carried out in bioreactors already for many years – is only now becoming more and more frequent on a shake-flask level due to advancing technologies. Recent developments have now led to the availability of a brand-new application called parameter-based feeding in shake flasks. Parameter-based feeding describes the feeding of a substance to a growing microbial culture, initiated when certain levels of a selected parameter (i.e. biomass or dissolved oxygen) are reached.
Multiparameter sensors and dissolved oxygen (DO) sensor pills for shake flasks
The ‘blind’ shake-flask optimizations helped to establish an expression setup that was then tested with the new DOTS Platform sensors by sbi.
Compared to bioreactors, shake flasks are a low-cost, easy-to-use option for use in small-scale lab settings
At the center of the DOTS Platform is the Multiparameter Sensor (MPS), a new optical sensor that is placed under the shake flask and measures a variety of parameters, including biomass and fluorescence, non-invasively through the vessel wall. At the same time, ambient sensors in the system measure temperature, shaking speed, humidity, and pressure online.

New dissolved oxygen (DO) pills can then be added to the medium. The pill is dropped into the culture, following the medium, and is paired with the MPS to measure dissolved oxygen levels in the culture.
In this next round of experiments, a PID-controller was also implemented in the methanol feeding system to trigger or stop feeding when a certain value of dissolved oxygen is reached. Here, a dissolved oxygen trigger point is set. As soon as the level of dissolved oxygen increases, the flow rate is calculated by the system, and a shot of methanol is fed. The cells respond quickly, consuming the feed. The level of dissolved oxygen decreases, and the feed is stopped.
The overall process seen using DO-based feeding matched that from the previous ‘blind’ optimization process. A similar feeding profile was observed, using the same levels of methanol in the feed, and similar levels of protein yield were achieved. Triggering methanol feeding into the cultures based on real-time DO measurements resulted in better control and reduced oxygen limitations in the flask, allowing for a higher protein yield and better comparability to bioreactor runs. In this round of experiments, however, these comparable results and additional process improvements were realized with several experiments in parallel through the course of one week.
A modern era of shake-flask bioprocessing
In conclusion, the continuous online acquisition of data from the DOTS Platform during different experiment runs provided critical information that helped to further characterize Dr Dickmeis’ bioprocess and improve yields. At the same time, the implementation of sensors and actuators reduced the need for manual sampling and interruptions, streamlining the process and significantly reducing the time required to achieve results. With modern technology, such as the DOTS Platform, shake flasks gain data access and control options and can now compete with advanced bioreactor processes, while staying low-cost, parallelizable, and easy to use.
• Tiffany Fredette spent more than 10 years in life sciences before joining sbi as a Product Marketer. Drawing from a diverse background, from molecular biotechnology to conservation biology, she now works to connect scientists with solutions
For more information visit www.scientificbio.com
If you have any questions or would like to get in touch with us, please email info@futureofproteinproduction.com


%20ILVO%202.jpg)

.png)

