
Cell-cultivated meat protein shows digestibility gains in new Food Chemistry study
Protein derived from cell-cultivated meat showed significantly higher digestibility and a greater potential to release bioactive peptides than conventional animal and plant proteins, according to a new peer-reviewed study published in Food Chemistry.
The research was led by Yunting Xie of Nanjing Agricultural University and colleagues, and compared the digestion behavior of cell-cultivated pork protein with traditional pork, soy, egg, whey, and casein using a standardized in vitro digestion model. One of the study’s co-authors, Shijie Ding, is CEO of cultivated meat company Joe’s Future Food.
The findings added new data to an area of alternative protein research that has so far focused more heavily on sustainability and scale than on nutrition and bioavailability.
Dietary protein plays a central role in maintaining tissue structure, metabolic function, and immune health. As global demand for animal protein continues to rise, interest in alternative production methods has intensified, driven in part by concerns over land use, greenhouse gas emissions, and water consumption associated with conventional livestock systems. Cell-cultivated meat, produced by growing animal cells outside the body, has been proposed as one pathway to address these pressures.
While environmental modeling has suggested potential benefits, comparatively little research has examined how proteins from cell-cultivated meat behave during digestion. Digestibility and amino acid availability are key indicators of protein quality, shaping how efficiently nutrients can be absorbed and utilized by the body.
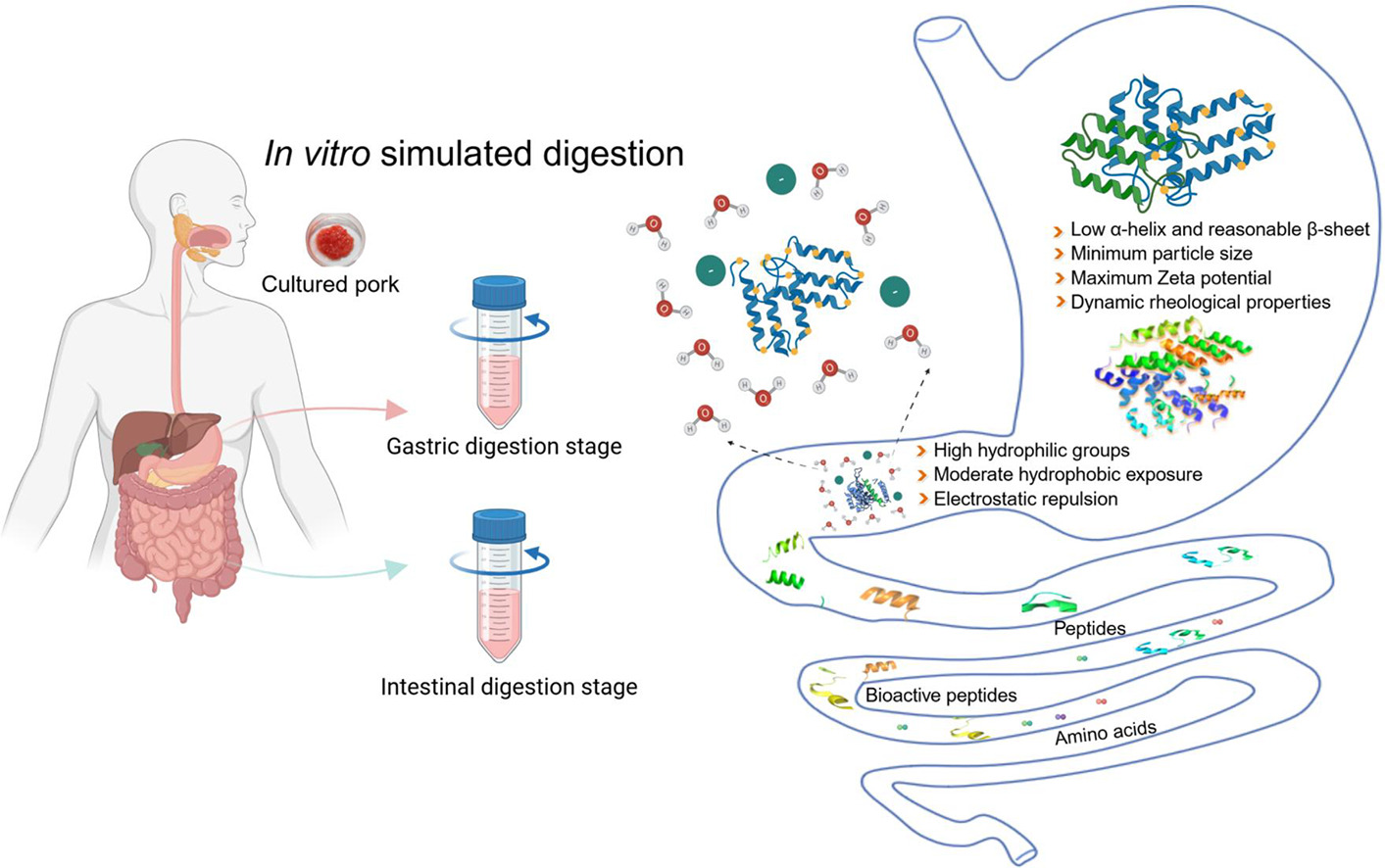
Using the INFOGEST 2.0 static digestion protocol, the researchers simulated gastric and intestinal digestion to assess protein breakdown. Cell-cultivated pork protein reached a digestibility of 76.77% during the gastric phase and 88.33% during the intestinal phase, outperforming traditional pork and all other protein sources included in the study.
Digestion products from cell-cultivated pork also contained the highest proportion of peptides smaller than 3 kDa, indicating more efficient enzymatic hydrolysis. LC-MS/MS analysis identified 3,626 peptides in gastric digesta and 1,734 peptides in gastrointestinal digesta, exceeding the number detected for egg white, whey, and casein.
Beyond peptide diversity, the study found that digestion of cell-cultivated pork released significantly higher levels of total amino acids and essential amino acids. Essential amino acids must be obtained through diet and are a critical benchmark for evaluating protein nutritional value.
Elliot Swartz, Senior Principal Scientist of Cultivated Meat at The Good Food Institute, said the findings addressed a long-standing question in the field. “A common critique of cultivated meat compared to other foods is the lack of knowledge around nutrition and bioavailability,” he said. “In the first study of its kind, to my knowledge, researchers found that cultivated pork had a higher protein digestibility and more free amino acids than conventional pork, casein, whey, soy, and egg white proteins, suggesting a high bioavailability for cultured meat.”
Swartz cautioned that the results should be interpreted carefully. “Results would need to be replicated in vivo, and there are some methodological and generalizability limitations,” he said, adding that the work nevertheless represented “an interesting set of findings”.
To understand why cell-cultivated pork performed differently, the researchers analyzed protein structure and the digestive microenvironment. Spectroscopic techniques indicated that the proteins exhibited structural characteristics that increased accessibility to enzyme cleavage sites. Measurements of particle size, zeta potential, and micro-rheological behavior suggested that digestion of cell-cultivated pork altered the digestive environment in ways that further supported enzymatic activity.
The authors contrasted these findings with known behaviors of other proteins. Whey protein typically undergoes rapid digestion due to its relatively simple structure, while casein forms micellar networks that slow amino acid release. Plant proteins such as soy may contain antinutritional compounds that reduce digestibility. In this context, cell-cultivated pork appeared to follow a distinct digestion pathway rather than mirroring existing animal or plant proteins.
The researchers described the study as the first to integrate digestion kinetics, peptide profiling, amino acid release, microenvironmental changes, and protein structural analysis for cell-cultivated meat. They suggested the results could support future work in precision nutrition and food formulation, while emphasizing the need for follow-up studies beyond in vitro models.
As cell-cultivated meat moves closer to commercialization, nutritional evidence is likely to play a growing role alongside environmental and economic considerations. While the findings were based on simulated digestion rather than human trials, the study provided detailed mechanistic insight into how cell-cultivated meat proteins may perform once consumed.
If you have any questions or would like to get in touch with us, please email info@futureofproteinproduction.com

.png)





.webp)
