
CRISPR breakthrough turns meat-like fungus into a faster, cleaner, more efficient protein source
A team of scientists in China has used CRISPR to transform a naturally meat-like fungus into a far more efficient and environmentally friendly source of protein, offering a potential boost for the growing mycoprotein category. Published in Trends in Biotechnology, the study demonstrated that the engineered strain of Fusarium venenatum grew faster, required significantly fewer inputs, and produced dramatically lower greenhouse gas emissions than its unmodified counterpart.
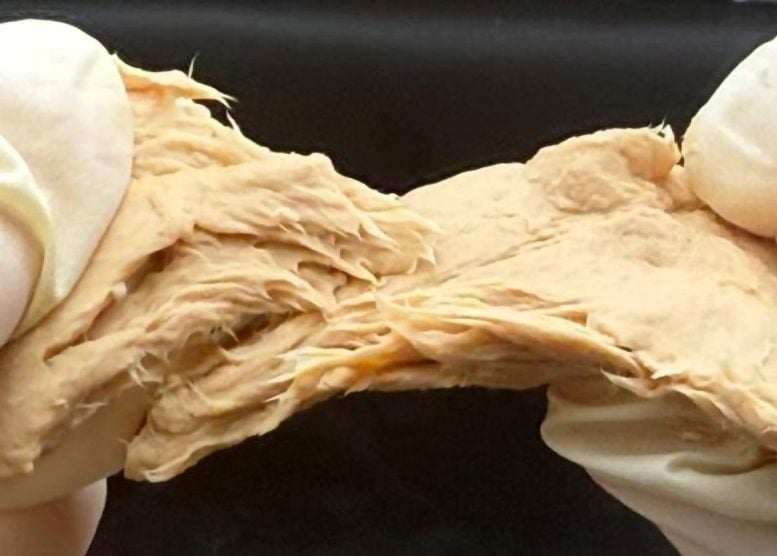
Fusarium venenatum is already one of the most established microbial protein sources on the market, valued for its fibrous structure and mild, meat-adjacent flavor. But despite its strengths, the organism has presented two longstanding challenges: a thick cell wall that limits digestibility and a resource-intensive production process that requires substantial sugar and nutrients. The research team at Jiangnan University set out to address both.
Led by corresponding author Xiao Liu, the group used CRISPR to delete two genes – one linked to chitin synthase, which affects the cell wall, and another tied to pyruvate decarboxylase, which influences metabolic efficiency. Removing the chitin synthase gene reduced the thickness of the fungal cell wall, making more of the underlying protein accessible to the human digestive system. Removing the second gene altered metabolic pathways so that the organism could produce protein using fewer nutrients.
“There is a popular demand for better and more sustainable protein for food,” Liu said. “We successfully made a fungus not only more nutritious but also more environmentally friendly by tweaking its genes.”
The resulting strain, named FCPD, showed striking gains in performance. According to the study, it used 44% less sugar to generate the same amount of protein as the wild strain, and it did so 88% more quickly. The researchers noted that this level of efficiency could meaningfully reshape the economics of mycoprotein production, which traditionally depends on large fermentation tanks fed with sugar and ammonium sulfate.
The team also emphasized that the CRISPR modifications did not introduce any foreign DNA into the fungus, potentially easing regulatory pathways in markets that treat gene editing differently from genetic modification. Fusarium venenatum itself is already approved for consumption in several major regions, including the United Kingdom, China, and the USA.
After demonstrating performance improvements in the laboratory, the researchers evaluated the environmental impact of FCPD at an industrial scale. They modelled production in six countries with varying energy systems, from Finland’s largely renewable grid to China’s more fossil-fuel-dependent mix. Across every scenario, FCPD outperformed the baseline strain. Overall, the team found a reduction of up to 60% in greenhouse gas emissions across the full production life cycle.
Co-author Xiaohui Wu said the industry had not fully explored how the production process itself could be optimized for sustainability. “A lot of people thought growing mycoprotein was more sustainable, but no one had really considered how to reduce the environmental impact of the entire production process, especially when compared to other alternative protein products,” Wu said.
The researchers also compared the performance of FCPD with conventional animal agriculture. When set against chicken production in China, the study found that FCPD-based mycoprotein required 70% less land and reduced the risk of freshwater pollution by 78%. These findings reinforce the long-discussed potential for microbial proteins to deliver high-quality nutrition with far lower resource demands.
As pressure grows on global food systems, microbial and fermentation-based proteins have received increasing attention as scalable alternatives to animal agriculture. The study’s authors argue that gene-edited strains could help unlock the next wave of efficiency and sustainability gains in the sector.
“Gene-edited foods like this can meet growing food demands without the environmental costs of conventional farming,” Liu said.
The work was supported by the Key Research and Development Program of China, the Jiangsu Basic Research Center for Synthetic Biology, the Natural Science Foundation of Jiangsu Province, and the Postgraduate Research & Practice Innovation Program of Jiangsu Province.
If you have any questions or would like to get in touch with us, please email info@futureofproteinproduction.com

.png)





.jpg)
