ETH Zurich breakthrough brings lab-grown beef closer to reality
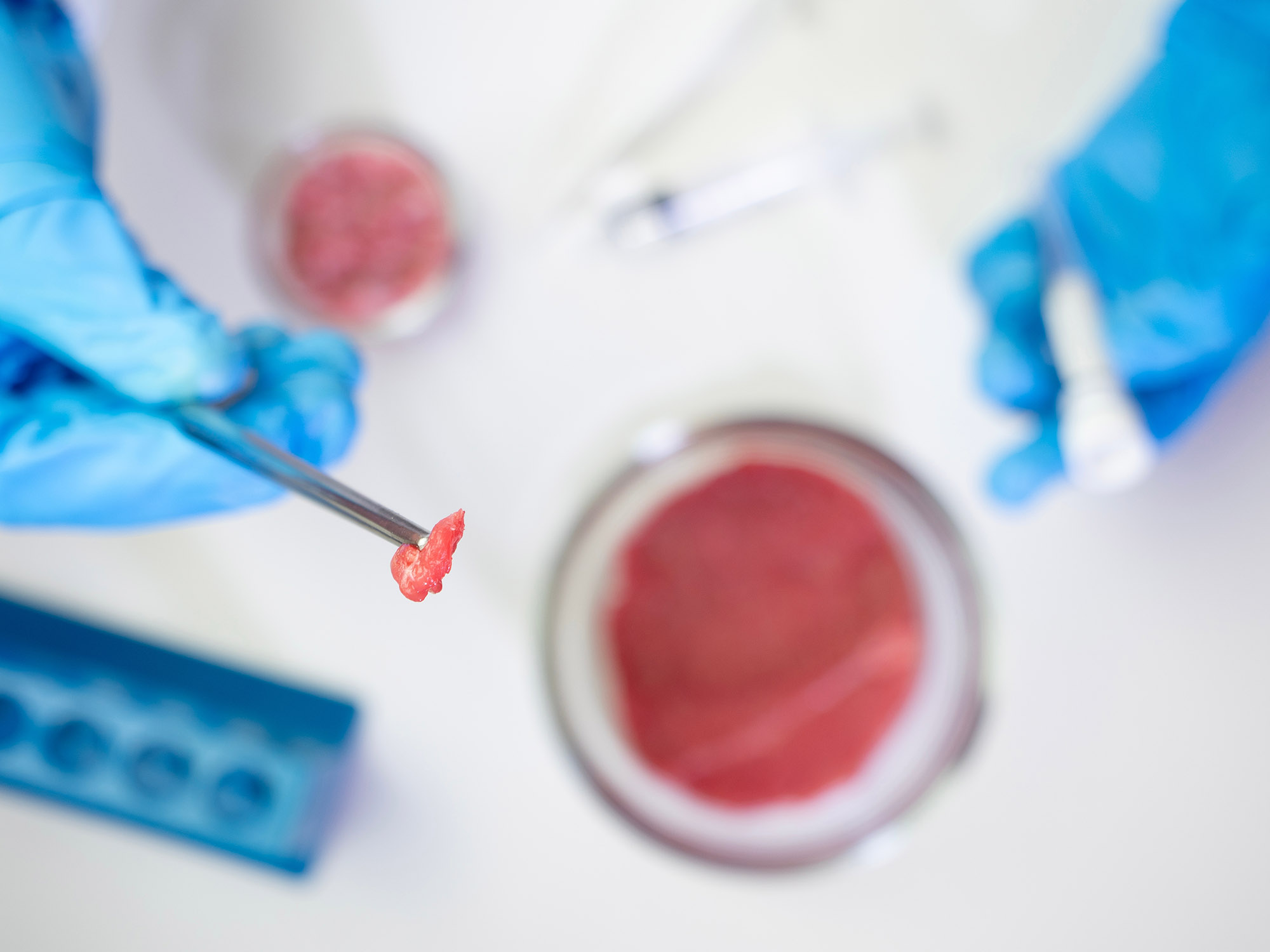
Scientists at ETH Zurich have achieved a significant milestone in the quest to create realistic lab-grown beef, cultivating thick, functional muscle fibers from bovine cells in a laboratory setting. The breakthrough, published by ETH Zurich on 29 July, could accelerate the development of cell-cultivated beef that looks, feels and tastes more like conventional meat.
Led by regenerative and muscle biology expert Professor Ori Bar-Nur, the team developed a novel method to grow three-dimensional muscle tissue from myoblasts – precursor cells that form muscle fibers. Although similar cells had been used in previous research, those efforts had typically resulted in much thinner, less realistic fibers.
The ETH research changes that. Using myoblasts extracted from standard beef cuts including fillet, sirloin, cheek and flank, the researchers managed to grow muscle tissue that mirrors natural bovine muscle at both the molecular and functional levels. The lab-grown tissue expresses the same genes and proteins and is capable of contracting like its naturally occurring counterpart.
The key to this success lies in a carefully designed cocktail of three molecules added to the nutrient-rich culture medium at the start of the growth process. “The cell culture medium requires further optimization to make it more affordable and safe for consumption. Additionally, we need to explore ways to produce these muscle fibers in larger quantities,” said Christine Trautmann, a doctoral student in Bar-Nur's group and one of the study’s lead authors.

While the use of molecular additives in food production may raise eyebrows, the ETH team notes that these compounds are used only during the early stages of cell development and are removed well before any final product would be harvested.
The approach builds on Bar-Nur’s earlier work at Harvard University, where he developed the molecular cocktail while researching muscle regeneration and therapies for hereditary muscle-wasting diseases in mice. That foundational research now appears to have applications far beyond the medical field.
So far, the team has produced only a few grams of muscle tissue. Scaling up production remains a major challenge, as does ensuring affordability and regulatory compliance. “These innovative new food products will have to undergo a prolonged and complex authorisation procedure before they reach shop shelves and, ultimately, our plates,” explained Adhideb Ghosh, the study’s other lead author and a scientist in Bar-Nur’s lab.
The research is supported by the Good Food Institute – a nonprofit focused on accelerating alternative protein development – as well as Swiss Food Research and Innosuisse. The findings come as the global race to commercialize cell-cultivated meat intensifies.
Start-ups and research groups around the world are working toward the goal of producing slaughter-free meat using cultured animal cells, with the potential to drastically reduce land use and eliminate the need for livestock transport and abattoirs. Singapore has already approved the sale of cultivated chicken, and other countries are exploring regulatory pathways.
Bar-Nur has yet to taste the cultivated beef himself, as Swiss law currently prohibits consumption of such products without official approval. However, he says colleagues who have participated in approved tastings describe the product as very close in taste and texture to conventional beef.
With further refinement, this research could lay the groundwork for the development of more authentic, scalable, and ethically produced beef alternatives. Bar-Nur is even considering launching a start-up to commercialize the technology.
“We want to help ensure that we will one day be able to produce ethically sound burgers that are affordable and safe,” he said.
If you have any questions or would like to get in touch with us, please email info@futureofproteinproduction.com