

Researchers develop chickpea protein scaffolds to advance cultured meat production
A team of scientists in Israel has unveiled a breakthrough in scaffold design for cultured meat production, demonstrating that chickpea protein can be transformed into edible structures capable of supporting cell adhesion and growth. The research, published in Food Hydrocolloids, introduces chickpea-based microcarriers and fibrous scaffolds as promising building blocks for sustainable, scalable cultivated meat.
One of the central challenges in cultured meat development is finding scaffolds that not only guide cell growth but are also food-grade, affordable, and free from animal-derived materials such as gelatin or collagen. While these biomaterials have long been used in tissue engineering, their reliance on animal inputs and regulatory hurdles limit their application in food. The new study highlights chickpea protein as a viable alternative, combining biological compatibility with consumer-friendly attributes.
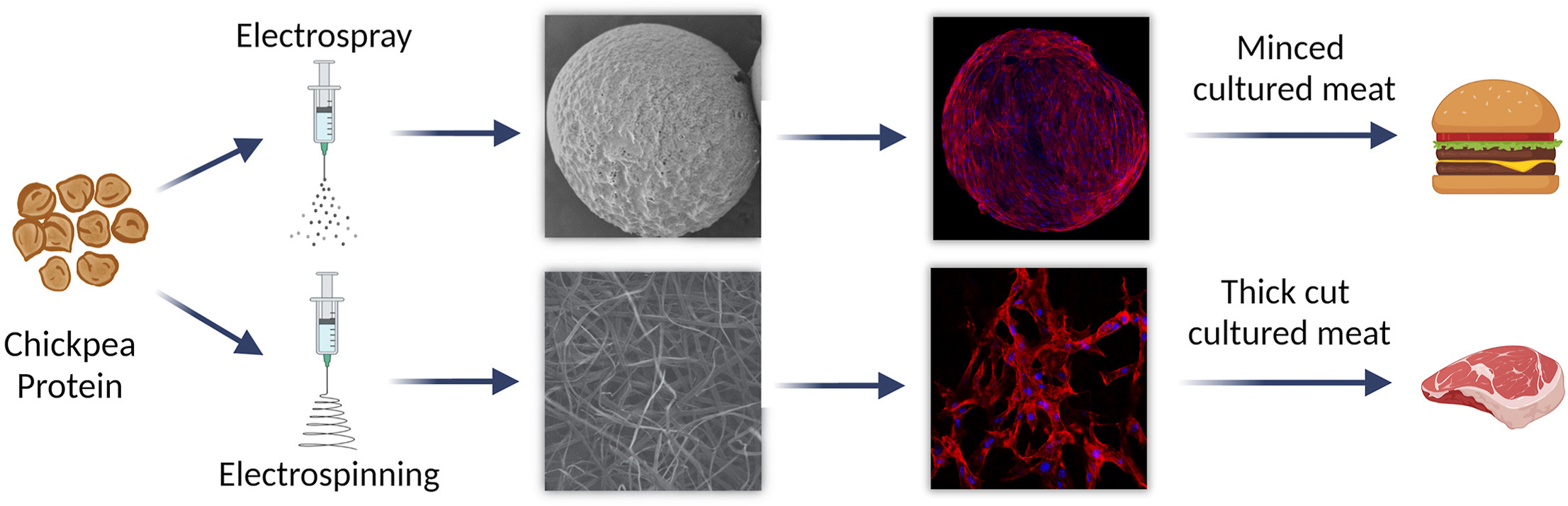
The researchers developed two distinct scaffold formats. The first, chickpea protein microcarriers, were produced using electrospray technology and solvent exchange to create spherical particles averaging 577 micrometers in diameter. These microcarriers are suitable for cultivating cells that can be used in minced or processed cultured meat products such as patties.
The second format, chickpea protein fibrous scaffolds, was created using electrospinning. This technique produced mats of fibers with an average width of 4.5 micrometers, either randomly aligned or oriented in one direction. Such fibrous scaffolds are particularly suited for growing thicker cuts of cultured meat, as the aligned fibers encourage skeletal muscle cells to organize in a structure similar to conventional meat.
Both scaffold types retained the nutritional and structural properties of chickpea protein through the fabrication process, ensuring they remain edible and potentially enriching the final cultured meat product.
In laboratory testing, chickpea protein showed no cytotoxic effects, with mesenchymal stem cells and skeletal muscle cells adhering and proliferating effectively. Within 10 days of culture, the chickpea microcarriers were fully covered by cells, while the fibrous scaffolds enabled an eightfold increase in cell expansion within a week. Notably, aligned fibers guided the orientation of skeletal muscle cells, an essential step toward replicating the texture of traditional meat.
These findings suggest chickpea scaffolds could help overcome one of the largest barriers in cultured meat development: creating edible, plant-based supports that deliver both functionality for cell culture and nutritional value for consumers.
Chickpeas are widely consumed globally, forming a staple of diets in the Mediterranean and Middle East and gaining popularity worldwide through products such as hummus and falafel. They are nutrient-dense, with a balanced amino acid profile, and are recognized as safe (GRAS) by regulators. From a technological perspective, chickpea protein offers useful properties including solubility, emulsification, gelling, and high water and oil absorption capacity.
These traits, combined with broad consumer familiarity, make chickpeas an attractive choice for scaffold development compared to more established legume proteins such as soy and pea. The study notes that while legumes have been used to supplement cultured meat scaffolds, creating fully plant-based, structurally stable scaffolds has been difficult due to limited solubility and cell adhesion properties. Chickpea protein, structured through electrospray and electrospinning, appears to address many of these limitations.
The research was carried out by scientists at the Technion – Israel Institute of Technology, drawing on expertise from both biotechnology and engineering. The team included Shira Levi, Anton Zernov, Limor Baruch, and Marcelle Machluf from the Faculty of Biotechnology and Food Engineering, alongside Patrick Martin and Eyal Zussman from the Faculty of Mechanical Engineering.
Machluf, a leading figure in tissue engineering, has long explored biomaterials for regenerative medicine and cellular agriculture, while Zussman is recognized for pioneering work in electrospinning and nanofiber technologies. Together with their colleagues, they combined knowledge of cell biology, protein chemistry, and advanced materials processing to design scaffolds that meet the dual demands of food safety and functionality.
The research also emphasizes the scalability of the technologies employed. Electrospray can be used to generate large quantities of edible microcarriers suitable for stirred tank bioreactors, while electrospinning offers a route to more complex fibrous scaffolds for premium products. While thick cuts produced on fibrous scaffolds may remain more expensive due to culturing complexities, microcarrier-based products could offer a cost-effective entry point for commercialization.
The ability to produce scaffolds entirely from chickpea protein reduces reliance on costly or non-food-grade inputs, aligning with the sector’s push toward affordability, sustainability, and consumer acceptance.
The study concludes that chickpea protein scaffolds present “impressive potential” for the development of edible, sustainable, and affordable cultured meat products. By preserving the nutritional benefits of chickpeas while enabling robust cell growth, these scaffolds could provide the foundation for future innovation in the field.
As demand for alternatives to conventional meat grows, plant-based scaffold materials are likely to play an increasingly critical role. With chickpeas already established in global diets and associated with health benefits such as weight management and improved metabolic regulation, their application in cultured meat could help bridge consumer familiarity with next-generation food technologies.
If you have any questions or would like to get in touch with us, please email info@futureofproteinproduction.com

.png)




.webp)

